Abstract
Introduction: Enhancer of zeste homolog 2 (EZH2) is the histone methyltrasferase catalytic subunit of the polycomb repressive complex 2 responsible for mono-, di-, and trimethylation of lysine 27 on histone 3. Tazemetostat, a potent, selective EZH2 inhibitor, is in phase 2 clinical development in relapsed or refractory (RR) Non-Hodgkin Lymphoma (NHL). Activating mutations in EZH2 are hypothesized to lead to an oncogenic EZH2 dependency. These mutations are most commonly detected in germinal center derived malignancies such as follicular lymphoma and the germinal center B-cell (GCB) subset of Diffuse Large B-cell Lymphoma (DLBCL). During B-cell maturation, EZH2 expression levels are highest in the germinal center and are then reduced following B-cell exit from the germinal center. Pre-clinical studies demonstrated that tazemetostat has greater anti-tumor activity in EZH2 mutant models as compared to EZH2 wild-type (WT) models. Given these data, during initial tazemetostat clinical development, it was anticipated that response rates in DLBCL patients would be highest in EZH2 mutant > EZH2 WT and GCB WT > non-GCB WT. Here we report the preliminary response rate data from the DLBCL cohorts of the ongoing phase 2 tazemetostat trial stratified by cell of origin (COO) using Hans immunohistochemistry (IHC) and NanoString's gene expression profiling (GEP) based Lymphoma Subtyping Test (LST).
Methods: As of June 1, 2017, the phase 2 single agent trial (NCT01897571) of tazemetostat had enrolled 142 RR DLBCL response evaluable patients (≥18 years old; ≥2 prior treatment regimens; measurable disease; and adequate organ function). Patients were allocated to cohorts based on the results from testing of archived tumor tissue using the cobas® EZH2 Mutation Test (Roche Molecular Systems, in development) designed to detect EZH2 hot spot activating mutations (Y646X, A682G, A692V) and IHC using the Hans methodology. Enrollment to the GCB- EZH2 WT and non-GCB cohort was completed in early 2017, while the GCB- EZH2 mutant cohort remains open. Retrospectively, COO was determined using NanoString's research use only version of the Lymph2Cx LST using RNA isolated from archived tumor tissue. Interim response rates for COO subsets using Hans IHC and LST methods were compared and contrasted using a Clopper-Pearson exact confidence interval. Hans classifies patients as GCB or non-GCB therefore, for the purposes of comparison, LST results of both activated B-Cell (ABC) and unclassified were considered to be non-GCB.
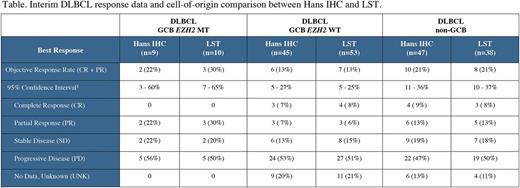
Results: Archived tumor from 101 of 142 evaluable DLBCL patients successfully generated data by both COO methods, with success primarily dependent on tissue availability for LST as only one patient sample failed LST data generation. Concordant COO classification was obtained for 89/101 patients (88%) with the majority of discordance due to switching to GCB by LST from non-GCB via Hans. The rate of COO concordance reported here between Hans and LST are in line with results from previously reported comparisons. Interestingly, three EZH2 mutant patients were enrolled in the non-GCB cohort however, via LST these patients were classified as one of each GCB, ABC and unclassified. This is the first known report of EZH2 mutations occurring in the ABC subtype. The number of response evaluable patients with EZH2 MTs is too small to make any conclusions regarding the impact of COO classification on response within such patients at this time. As shown in Table 1, within the EZH2 WT patients, no significant difference in interim objective response rate (Cheson 2007) between the GCB vs. non-GCB subsets was observed using either Hans or LST classifications.
Conclusions: The current interim DLBCL response data from patients with EZH2 MTs in this ongoing phase 2 tazemetostat study has insufficient patient numbers to effectively assess the impact of COO on response in these patients. Intriguingly, in EZH2 WT patients to date, COO does not appear to be a primary driver of response prediction indicating that other factors independent of COO may be more predictive of single agent tazemetostat activity in these patients.
McDonald: Epizyme, Inc: Employment, Equity Ownership, Research Funding. Morschhauser: Janssen: Consultancy, Honoraria; Servier: Consultancy; Celgene: Consultancy, Honoraria; Gilead: Consultancy; Roche: Consultancy, Honoraria; Bristol-Myers Squibb: Consultancy, Honoraria. Ribrag: Esai: Honoraria, Research Funding; Pharmamar: Consultancy; Servier: Consultancy, Honoraria; ArgenX: Research Funding; Roche: Honoraria, Other: travel, accommodation, expenses; BMS: Consultancy, Honoraria; MSD: Consultancy, Honoraria; Infinity: Consultancy, Honoraria; Gilead: Consultancy, Honoraria; Nanostring: Consultancy, Honoraria; Epizyme: Consultancy, Honoraria. Salles: Morphosys: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Amgen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Roche: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; BMS: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Merck: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Gilead: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Servier: Consultancy, Honoraria; MSD: Consultancy, Honoraria; Kite: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees. Tilly: Roche: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Karyopharm: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Takeda: Consultancy, Honoraria; Immunogen: Honoraria; Gilead: Honoraria; Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees. Gerecitano: Arcus Medica: Consultancy, Membership on an entity's Board of Directors or advisory committees; Bayer: Consultancy, Membership on an entity's Board of Directors or advisory committees; Genentech: Honoraria, Membership on an entity's Board of Directors or advisory committees; Samus Therapeutics: Honoraria, Membership on an entity's Board of Directors or advisory committees; Abbvie: Honoraria, Membership on an entity's Board of Directors or advisory committees; Gilead: Honoraria, Membership on an entity's Board of Directors or advisory committees; Mass Medical International: Honoraria, Membership on an entity's Board of Directors or advisory committees; Merck: Honoraria, Membership on an entity's Board of Directors or advisory committees; Aratana: Consultancy, Membership on an entity's Board of Directors or advisory committees; Incyte: Consultancy, Membership on an entity's Board of Directors or advisory committees; Orexo: Honoraria, Membership on an entity's Board of Directors or advisory committees; Royal Bank of Canada: Honoraria, Membership on an entity's Board of Directors or advisory committees. Dickinson: GlaxoSmithKline: Honoraria, Research Funding. Assouline: Novartis Canada Inc.: Honoraria; Bristol Myer Squibb: Speakers Bureau; Pfizer: Speakers Bureau; Paladin: Speakers Bureau; Janssen: Honoraria. Haioun: Roche: Consultancy, Honoraria; Amgen: Consultancy, Honoraria; Takeda: Consultancy, Honoraria; Sandoz: Consultancy, Honoraria; JANSSEN: Consultancy, Honoraria; GILEAD: Consultancy, Honoraria; PFIZER: Consultancy, Honoraria. Gribben: Genentech/Roche: Honoraria; Karyopharm: Honoraria; Acerta: Honoraria; TG Therapeutics: Honoraria; Janssen: Honoraria; Kite: Honoraria; Pharmacyclics: Honoraria; Celgene: Honoraria; Abbvie: Honoraria. Zinzani: Gilead: Other: Advisory board; Takeda: Other: Advisory Board; Janssen: Other: Advisory board; Celgene: Other: Advisory board; Roche: Other: Advisory board; Sandoz: Other: Advisory board; Pfizer: Other: Advisory board; Karyopharma: Other: Advisory board. Wu: Roche: Employment, Equity Ownership. Kussick: PhenoPath: Employment, Other: part owner; Seattle Genetics Hematopathology Advisory Board: Honoraria. Storhoff: NanoString Technologies: Employment, Equity Ownership. Larus: Epizyme, Inc: Employment, Equity Ownership. Clawson: Epizyme, Inc: Employment, Equity Ownership. Grayson: Epizyme, Inc: Employment, Equity Ownership. Daigle: Epizyme, Inc: Employment, Equity Ownership. Ho: Epizyme, Inc: Employment, Equity Ownership. Miao: Epizyme, Inc: Employment, Equity Ownership. Blakemore: Epizyme Inc.: Employment, Equity Ownership.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal